Abstract
Background:
Diffuse large B cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma, with an estimated incidence of 5.6 cases per 100,000 Americans annually. The standard of treatment for relapsed DLBCL is salvage chemotherapy with autologous stem cell transplantation (auto-SCT). However there remains a paucity of data regarding outcomes in minority racial/ ethnic groups or patients of low socioeconomic status. Our institution primarily serves the population of Bronx County, NY, where 24.4% of individuals live at or below poverty level and 8.5% are uninsured.
Patients and Methods:
We conducted a retrospective analysis from a single academic center in the Bronx, NY with identification of patients with relapsed DLBCL treated with auto-SCT from 2005 to 2020. Patients were excluded if they had primary refractory disease or if they achieved partial or complete remission with first line chemotherapy. A review of individual cases was conducted to evaluate patient demographics, treatment history and survival post- transplantation. We generated survival curves (Kaplan Meier) for the overall population with subgroup analyses based on age, sex, race/ ethnicity, insurance and line of treatment, compared using log-rank test.
Results:
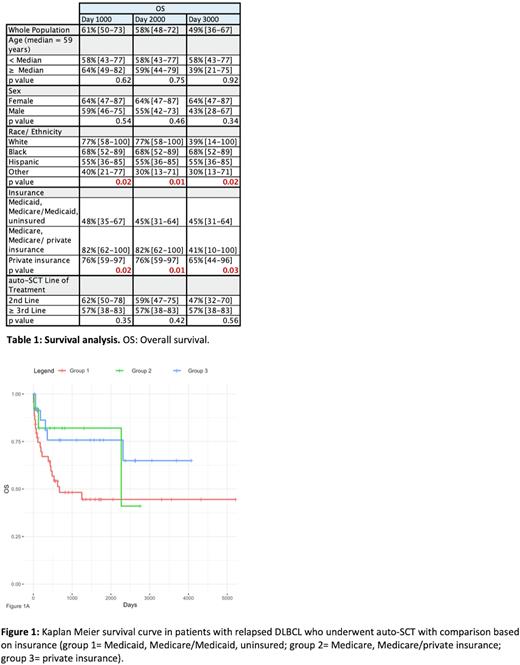
We identified 81 patients meeting inclusion criteria. The median age of patients was 59 years (range 23-79 years), with 59% male and 41% female. Of the 81 patients, 19% were White/ Caucasian (n= 15), 32% Black/ African-American (n= 26), 27% Hispanic/ Latinx (n= 22), and 22% other/ unidentified (n= 18). In terms of insurance coverage, 52% of patients had Medicaid coverage (either alone or combined with Medicare), 30% had private insurance, 16% had Medicare (either alone or combined with private insurance), and 2% were uninsured/ unidentified. Patients underwent auto-SCT after second line chemotherapy (72%, n= 58), third line (25%, n= 20), or fourth line (3%, n= 3).
Overall survival (OS) in this cohort was 61% vs 58% vs 49% at 1000, 2000, and 3000 days, respectively. Subgroup analyses based on age and sex did not show a significant difference. Similarly, patients who underwent auto-SCT after second line chemotherapy had similar OS relative to those who underwent transplant after third or fourth lines. However, there was a significant difference noted between OS of different racial/ ethnic groups with OS of 77% (CI 58-100) in White/ Caucasian patients, 68% (CI 52-89) in Black/ African-American patients, 55% (CI 36-85) in Hispanic/ Latinx patients, and 40% (CI 21-77) in other or unidentified ethnic groups at 1000 days (p= 0.02). There was also a significant difference in OS between patients with Medicaid coverage or uninsured (48%, CI 35-67) vs private insurance (76%, CI 59-97) vs Medicare without Medicaid (82%, CI 62-100) at 1000 days (p = 0.02).
Conclusion:
Our data suggests that for chemo-sensitive relapsed DLBCL, auto-SCT is associated with 61% OS at day 1000, similar to prior literature. However, despite access to stem cell transplantation for treatment of relapsed DLBCL, patients of racial/ ethnic minority groups or low socioeconomic backgrounds (as evidenced by Medicaid eligibility) have significantly reduced overall survival at 1000-, 2000-, and 3000-days post-transplantation compared to their counterparts. These findings suggest that socioeconomic disparities continue to impact delivery of oncologic and transplant care. A limitation of this study is sample size; future investigation into larger datasets of socioeconomically and racially diverse populations can provide further insight into auto-SCT outcomes.
Disclosures
Bazarbachi:ASH: Research Funding. Shastri:Janssen: Consultancy; Rigel Pharmaceutical: Membership on an entity's Board of Directors or advisory committees; NACE: Honoraria; Kymera Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Konopleva:AbbVie, Genentech, F. Hoffman La-Roche, Stemline Therapeutics, Amgen, Forty-Seven, Kisoji; Janssen: Consultancy; Stocks, Reata Pharmaceuticals: Current equity holder in publicly-traded company; AbbVie, Genentech, F. Hoffman La-Roche, Eli Lilly, Cellectis, Calithera, Ablynx, Stemline Therapeutics, Agios, Ascentage, Astra Zeneca; Rafael Pharmaceutical; Sanofi, Forty-Seven: Research Funding; Forty-Seven; F. Hoffman LaRoche: Honoraria; Reata Pharmaceuticals, Novartis and Eli Lilly: Patents & Royalties; Stemline Therapeutics, F. Hoffman La-Roche; Janssen: Membership on an entity's Board of Directors or advisory committees. Sica:PER Physician's Education Review.: Consultancy, Honoraria; MorphoSys: Consultancy, Honoraria; Miragen: Consultancy, Honoraria; Kite Pharma: Research Funding; Bristol Myers Squibb Foundation: Research Funding; AstraZeneca: Honoraria, Research Funding; Curios: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal